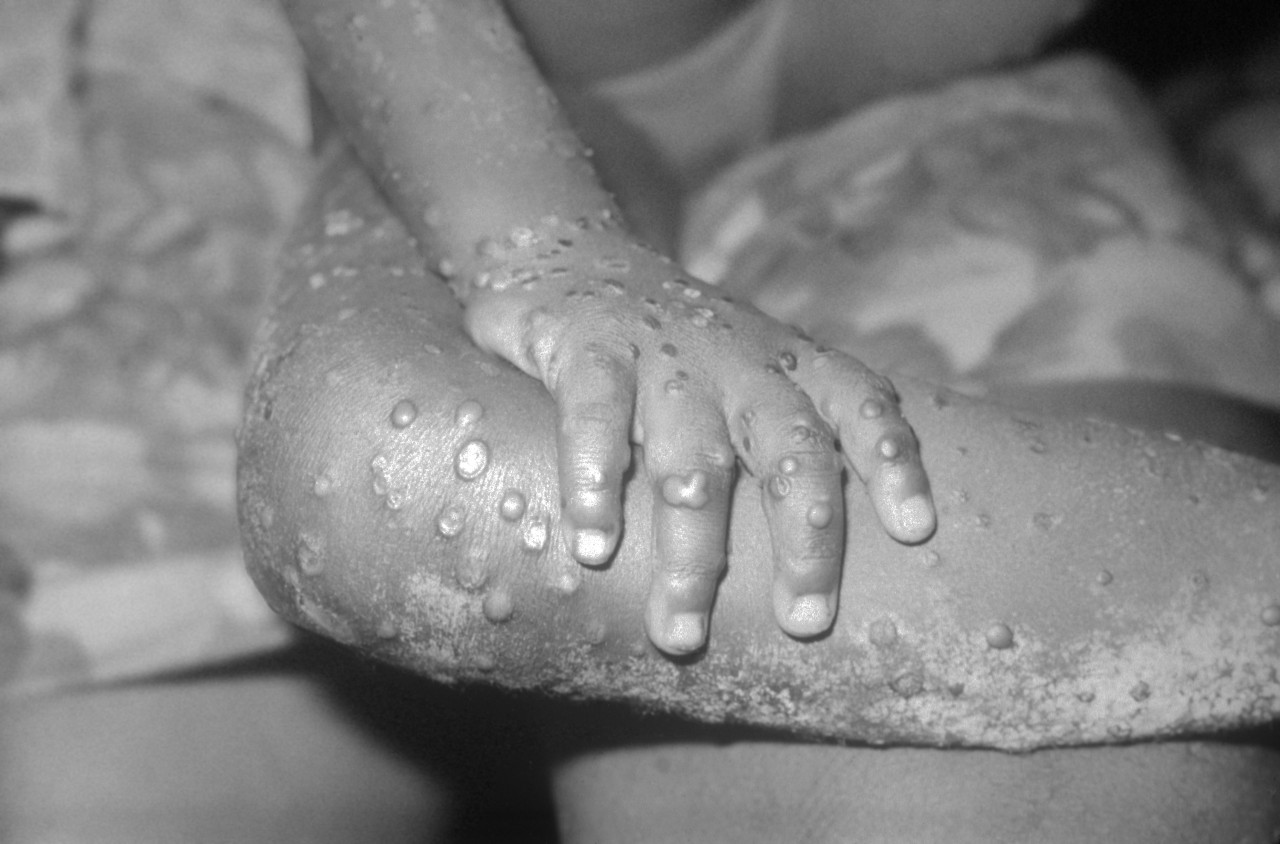
What we know about the decision to change the mode of administration of the smallpox vaccine to allow more people to be vaccinated
The European Medicines Agency (EMA) has authorised the administration of monkeypox vaccine intradermally rather than subcutaneously. This will allow more people to be vaccinated in the absence of doses, but effectiveness and safety data are limited. The Public Health Commission has decided to follow this strategy except for pregnant women and immunocompromised persons.