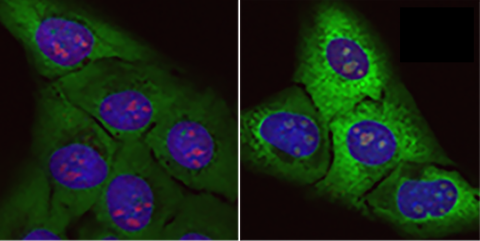
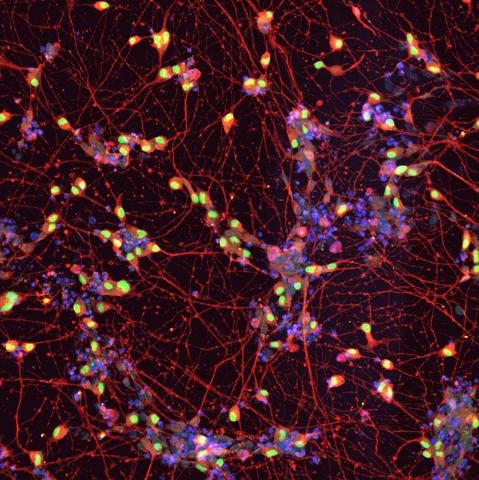
"Promising" new strategy for ALS using stem cells created from patients
Researchers in the United States have used stem cells created from patients with a very rare type of ALS, more prevalent in Brazil, to target a key gene in the stress response and reverse the damage suffered by motor neurons in the laboratory. They believe it is "a promising proof-of-concept for future therapeutic strategies" and "could help lay the foundation for genetically informed clinical trials".

Adolfo López de Munain - ELA Brasil
Adolfo López de Munain
Clinical Head of Neurology at the University Hospital of Donostia, Scientific Director of CIBERNED and Research Director of the Neurosciences area of ISS Biodonostia
The study is solid and based on research conducted on cells derived from iPS [induced pluripotent stem] cells from patients with this mutation in the VAPB gene. This approach has its limitations, as it is an artificial recreation of what occurs in vivo, but it is a good approximation for elucidating mechanistic aspects.
This study highlights three things:
- That ALS is not a homogeneous entity from a pathophysiological point of view and that, in this case, the fundamental role leading to motor neuron neurodegeneration lies in the connections between the mitochondria and the endoplasmic reticulum (MAMs).
- That there is, as a consequence of the dysfunction of these structures, an alteration in the integrated response to stress.
- That this may lead to therapeutic approaches with inhibitors of this IRS such as ISRIB or similar. In this regard, there are previous studies by Spanish researchers (Bugallo et al.) that already pointed in this direction.
The limitations stem from the model. The authors study this in derived motor neurons, assuming that this disorder occurs in vivo in these cells, ignoring the possibility that similar mechanisms may exist in glial support cells. It would be important to analyse the effect of the mutation in muscle and glial cells to see the effect through conditioned mutation in a single cell line in other animal models.
This finding opens the door to developing drugs with this target and also to attempting to analyse in a clinical context the weight of this mechanism in cases of sporadic ALS in order to stratify patients in trials. Unfortunately, in sporadic ALS, various mechanisms converge, and we do not know the individual weight of each of them and their chronological sequence in specific patients, which makes it difficult to establish a therapeutic plan adapted to the real context of each patient. To advance precision medicine in ALS, we must be able to weigh the real situation of each patient using biomarkers linked to each mechanism.
Juan Alberto Ortega Cano - ELA Brasil
Juan Alberto Ortega Cano
Ramón y Cajal Research Professor, Department of Pathology and Experimental Therapeutics, Faculty of Medicine and Health Sciences
The recently published work by Landry et al. focuses on a familial form of amyotrophic lateral sclerosis (ALS), the most common form in Brazil, using motor neuron models derived from human iPSCs [induced pluripotent stem cells]. It is a classic and robust study, cleverly using genetic methods to study the effect on motor neurons of the mutated protein in patients, VAPB, in its ‘healthy’ or mutated (P56S) form associated with ALS type VIII. In general, the conclusions are well supported by solid data including functional, molecular and morphological analyses, as well as phenotypic rescue through pharmacological inhibition of the integrated stress response (ISR).
Among the pathological phenotypes observed in motor neurons expressing only the mutant form are a reduction in the endoplasmic reticulum-mitochondrial connection, a reduction in mitochondrial membrane potential, and a reduction in motor neuron electrical activity. The authors coherently integrate existing evidence on the role of VAPB in the connection between the endoplasmic reticulum and mitochondria and the emerging role of ISR in neurodegeneration. The novelty of the study is the direct association it makes between mutation-specific mitochondrial dysfunction, due to reduced contact with the endoplasmic reticulum, and increased activation of ISR. Some of these phenotypes (not the electrophysiological one) have also been observed in motor neurons derived from iPSCs obtained from patients (with the mutation in heterozygosity) compared to controls where the mutation is genetically corrected. This corroborates that these defects can also occur in patients.
It remains to be understood how, mechanistically, the reduction in contact between the endoplasmic reticulum and mitochondria caused by the mutation generates greater stress in these compartments, leading to higher ISR activation. In fact, the role of ISR in ALS has been studied in recent years in multiple familial forms of the disease. According to the authors of the study, it appears that, in different forms of ALS, elevated ISR activation may have both positive and negative effects. Therefore, the use of drugs that modulate this pathway upwards or downwards will depend on the type of ALS patient. In fact, two clinical trials cited in the article, which enhance ISR activation, have already failed in the early stages.
Among the limitations, it is recognised that the results focus on a specific genetic subtype of ALS, which represents a very low percentage of patients (<1%), thus limiting immediate generalisation to other subtypes. Furthermore, although phenotypic reversal is demonstrated with an ISR pathway inhibitor, it is not fully explored whether treatment can halt or reverse neuronal degeneration in advanced stages.
The clinical implications are clear: this work supports the stratified treatment of ALS based on patient genetics and proposes ISR modulation as a viable therapeutic target in specific subgroups. This is particularly relevant following the failure of clinical trials with ISR inhibitors in unstratified populations. Furthermore, it should be studied in depth how these treatments can correct motor neuron degeneration without affecting how the rest of the body's cells deal with cellular stress.
David Pozo Pérez - ELA Brasil
David Pozo Pérez
Professor of Biochemistry and Molecular Biology at the University of Seville, principal investigator at CABIMER (CSIC-US) in the Laboratory of Cellular and Molecular Neuroimmunology
Research led by a team at Case Western Reserve University in Cleveland, Ohio, has identified the molecular effects of the P56S mutation on motor neuron function. They link this effect to inadequate responses of neurons to a stressful environment in which interactions between the mitochondria and the endoplasmic reticulum may play a significant role. This mutation is related to a rare subtype of ALS, called ALS Type VIII, which is characterised by its early onset (in the thirties) and very slow progression of motor impairment.
The work is important because it identifies mechanisms of action, which is essential for establishing rational strategies for pharmacological intervention. There are several limitations in terms of translating the results. The most important is that all the results were obtained in vitro and, therefore, the contribution of other cell types to the onset and establishment of this type of ALS is not contextualised. The absence of preclinical studies in humanised models would be of great added value. Molecular studies that help us understand how neurons manage stressful situations are essential, although we still do not know the origin, dynamics and effects of the causes that generate these situations.
If we know anything about the pathological mechanisms of ALS, it is that it is not a single entity and that dialogue between different cell types is essential to understanding it and, therefore, to managing it clinically in the future.
Alberto García Redondo - ELA células madre
Alberto García Redondo
Researcher at GenELA - Genetic Diagnosis and ALS Research Laboratory, 12 de Octubre University Hospital, 12 de Octubre Hospital Health Research Institute, Biomedical Research Network Center for Rare Diseases (CIBERER)
The study is of very good quality and is backed by data that can be replicated... although I don't know if this is sufficiently robust.
This is a very specific study, conducted in a cell model derived from patients (IPSCs), and we all know that these models are not very reproducible. This does not invalidate it, but we must consider that its reproducibility is in question in view of further investigation of the results.
In principle, it fits quite well with the existing evidence. It offers relatively novel data on the functionality of a protein encoded by the VCP gene. This gene gives rise to a very low percentage of familial ALS cases that are specifically limited to Brazil and other Portuguese-speaking countries (some families have been described in Mozambique). Around 0.01-0.05% of ALS patients (or even lower).
However, the data it provides on how the interaction process between the endoplasmic reticulum and mitochondria is altered offers new insights into a weak point in motor neurons, which could somehow lead to their degeneration and the neurodegenerative process in ALS. And, therefore, how the Integrated Stress Response (ISR) mechanism is altered in this type of neurodegeneration.
All of this provides new ideas for exploring novel avenues that may lead to the development of plausible therapies in the rather distant future. These therapies will undoubtedly be dedicated to a very specific subgroup of patients.
[On its limitations]
The results of the study are confusing in themselves. On the one hand, because the model used is not very reproducible and, above all, because the results are apparently promising, and the only thing they would demonstrate (if everything is proven to be reproducible in the long term and in other in vivo models) is a new pathway for the initiation and development of the neurodegenerative process in ALS.
[On the implications for the real world, for clinical practice]
At present, none. Looking ahead to the future, which is unfortunately still far off, it may be possible to test some types of therapies targeting this new mechanism supposedly associated with ALS, which could probably (always in the conditional, as these are future hypotheses) yield results in very specific and small subgroups of patients.
Landry et al.
- Research article
- Peer reviewed
- Experimental study
- In vitro