Continued use of melatonin for insomnia is associated with an increased risk of heart failure
Taking melatonin supplements to treat insomnia for more than a year is associated with an increased risk of heart failure—including an increase in hospitalisations and mortality—within five years of use, according to a study presented at a conference of the American Heart Association. The study compared a group of 65,000 adults diagnosed with insomnia who had a prescription for melatonin with another group who did not have a prescription for this supplement.

251103 melatonina carlos EN
Carlos Javier Egea Santaolalla
President of the Spanish Federation of Sleep Medicine Societies, coordinator of the Sleep Alliance Health Group, head of the Pulmonology Service and the Functional Sleep Unit of the Bioaraba Health Research Institute, coordinator of the SEPAR 2025-2026 year of sleep disorders and associate professor at the Faculty of Medicine of the University of the Basque Country
Our understanding of melatonin as a treatment is based on reviews and meta-analyses, such as a recent one (2025) published by Daliri et al., which confirms in a systematic review the positive effect of melatonin on patient outcomes, in this case, in patients with heart failure, where the authors consider melatonin to be a new treatment for these cardiac patients, even in palliative care.
Its most precise indication is for jet lag, chronic insomnia in people over 55, and children with autism spectrum disorder. The current abstract by Ekenedilichukwu Nnadi et al. is an observational study based on data recorded in a UK and US database (TriNetX Global Research Network for adults ≥ 18 years with an insomnia diagnosis (ICD-10 F51.0)), which seriously questions the results of the reviews, associating an 89% higher risk of heart failure and hospitalisation with one year of melatonin use compared to patients who did not take melatonin.
There are clear limitations to the study, based on the fact that it is an observational study, knowing in advance that these studies only show association and do not establish causality. Furthermore, given that melatonin does not require a prescription in the US, it is possible that the control group (without melatonin) included many patients who take melatonin without a prescription (the percentage is unknown, which could be a very significant bias) and is not reflected in their medical records. Finally, it is an abstract from a conference and, unlike publications in indexed medical journals, has not been filtered by two independent reviewers.
Therefore, these findings challenge the perception of melatonin as a benign chronic therapy and only highlight the need for a prospective trial with a control group to clarify its safety profile.
251103 melatonina javier EN
Javier Garjón Parra
Head of the Medicines Advisory and Information Service in the Pharmacy and Benefits Sub-Directorate
Melatonin is a hormone in the body that regulates sleep-wake cycles. For this reason, it is the active ingredient in prescription medications (Circadin®, Melatonina EFG) indicated for insomnia in adults over 55 years of age. However, as it is considered a safe substance, it can also be marketed as a food supplement and sold freely in the European Union. This regulation is somewhat surprising since, as a hormone, it performs functions in various parts of the body and may have unknown adverse effects. The safety of prolonged use of melatonin has not been studied in clinical trials. Therefore, this study, which examined whether long-term use of melatonin increases the risk of heart failure, is welcome. They use real-life data from the medical records of patients from several countries who were followed up. The method seems appropriate and is typical of drug safety studies.
It was observed that users of melatonin for one year or more had a risk of developing heart failure over five years that was almost double that of those who did not take it. This result could be expressed as follows: of every 100 patients in the study who took melatonin, two who developed heart failure would not have developed it if they had not taken it.
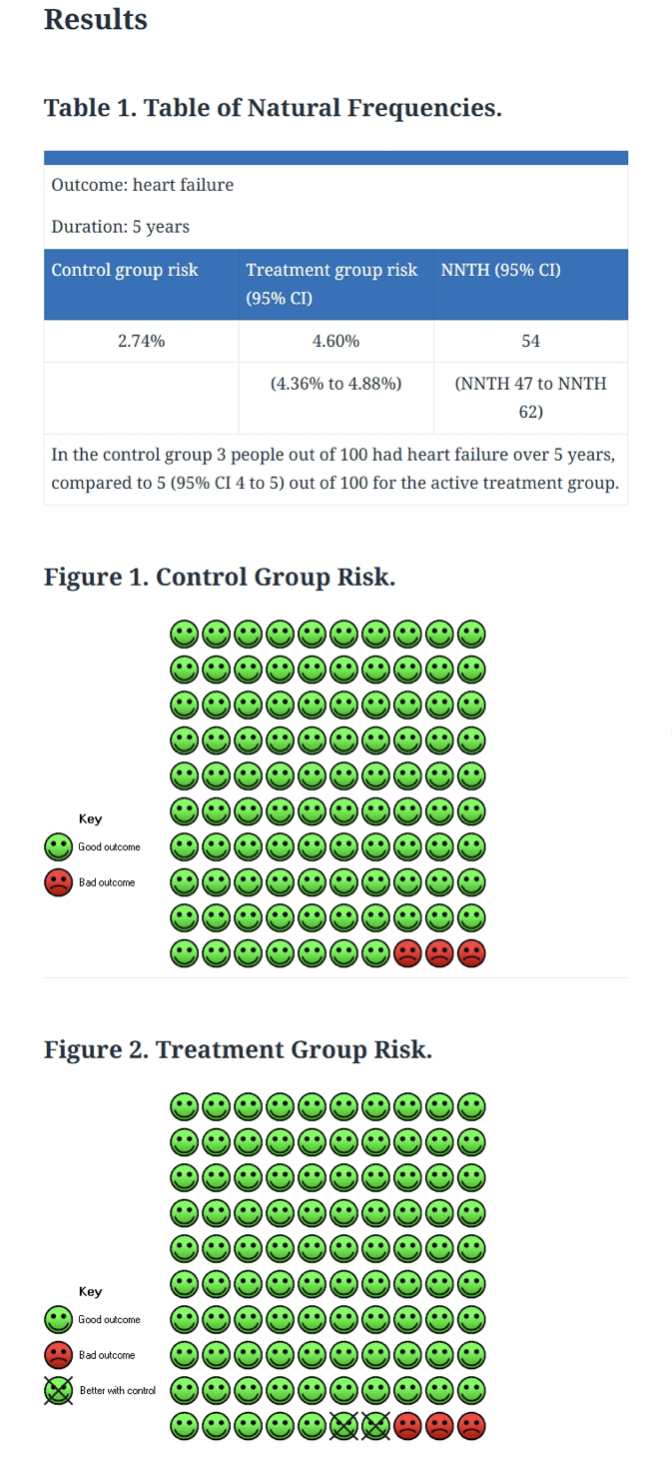
The strength of the study lies in the large number of patients included. It also lies in the fact that each melatonin user was matched with a patient with similar clinical characteristics. However, an inherent limitation of this type of study is that there could be some unknown variable that made patients both more likely to consume melatonin and more susceptible to heart failure.
This study should prompt regulatory bodies to consider whether it would be appropriate to restrict the marketing of melatonin to medicines. The European Medicines Agency should evaluate this study and, if appropriate, include a warning in the information on medicines containing melatonin.
In short, this is an important study on the risks of a product about which there is insufficient information and which should be evaluated by regulatory agencies. It certainly calls into question the claimed cardiovascular safety of melatonin.
Óscar Larrosa - melatonina insomnio EN
Óscar Larrosa
Clinical neurophysiologist, expert in sleep medicine and clinical care manager of the Sleep Medicine Unit at MIP Salud-Personalised Comprehensive Medicine
It is a case study (melatonin intake) versus control (no intake) with a large number of subjects studied over a long period of time, taking numerous variables into account. It is well designed according to the data in the summary of the study. The conclusions in these studies are that a relevant finding is linked to another with statistical significance. This does not imply that the finding is necessarily caused by the other (taking melatonin for at least a year); simply that they coincide, but they raise alarm bells. As the authors rightly say, it would be advisable to conduct more well-designed clinical trial-type studies to try to confirm a closer causal relationship, but I find it very relevant. We will have to wait until the full study is available to see the possible relationship with melatonin doses taken. If this has been taken into account, it could be an important piece of information.
Context: the discussion about the timing and dosage of melatonin is a hot topic among specialists worldwide, with advocates of long-term and/or high-dose use and detractors who advise caution in both cases due to the lack of reliable data on the subject.
Melatonin is a neurohormone that is responsible for the onset of the sleep window that promotes the onset of night-time sleep. It is secreted between one and three hours before the onset of this window under normal conditions, with the right environmental circumstances, by stimulating its brain receptors. However, it is also known that melatonin receptors are present in many other organs of the body, including the heart. The effects of stimulating these receptors have been linked to numerous functions, such as antioxidant and immune effects and the probable regulation of the functioning of these organs at the level of the circadian biological rhythm. It has not been proven that high doses of melatonin are more helpful in inducing night-time sleepiness, and the possible long-term effects on the brain are not well understood, including the possible saturation-inhibition of brain melatonin receptors. It is therefore recommended that it be taken continuously for a short period of time, i.e. a few months.
The high or very high doses recommended by its advocates, and/or long-term use, are mainly linked to its possible beneficial effects on other organs at the antioxidant and immune levels, including in cancerous diseases, with promising but still insufficient evidence, especially in terms of tolerance and possible harmful effects. The alleged safety of melatonin in the long term and/or at high doses is still not entirely confirmed at present. This work could change many things in these perceptions, if it is confirmed and replicated in other clinical trial-type studies. As an initial finding in many subjects and in the long term, it is very relevant and will have a profound impact.
Milagros Merino - melatonina insomnio EN
Milagros Merino Andreu
Specialist in Clinical Neurophysiology and coordinator of the Neurological Sleep Disorders Unit at La Paz University Hospital
Melatonin is a product that, although not without adverse effects, is usually very well tolerated, with a risk-benefit ratio in favour of its use, even in children.
Therefore, the abstract of this study is surprising, as it shows results not previously described and which raise doubts:
- It is an observational study (with its limitations).
- It only mentions an association, with no apparent evidence of a causal relationship between melatonin use and subsequent heart failure.
- It is unclear whether the control group were melatonin users without medical supervision, given the easy access to this product in some countries.
- This is a study presented at a scientific meeting, whose evaluation (by a scientific committee) is not as strict as the peer review (by two independent reviewers) carried out by scientific publications.
- It contrasts with the conclusions of recent meta-analyses regarding the use of melatonin, even in ICUs (Kelleher et al-PLoS One, Tang et al 2025, Darily et al 2025, the latter even describing an improvement in cardiac function).
However, these discrepancies are beneficial to science and raise a very positive debate, with the need for prospective studies with a “well-controlled” control group to ensure the safety profile of melatonin.
Ekenedilichukwu Nnadi et al.
American Heart Association Scientific Sessions 2025.
- Non-peer-reviewed
- Communication
- Observational study
- People



