Javier Garjón Parra
Head of the Medicines Advisory and Information Service in the Pharmacy and Benefits Sub-Directorate
Melatonin is a hormone in the body that regulates sleep-wake cycles. For this reason, it is the active ingredient in prescription medications (Circadin®, Melatonina EFG) indicated for insomnia in adults over 55 years of age. However, as it is considered a safe substance, it can also be marketed as a food supplement and sold freely in the European Union. This regulation is somewhat surprising since, as a hormone, it performs functions in various parts of the body and may have unknown adverse effects. The safety of prolonged use of melatonin has not been studied in clinical trials. Therefore, this study, which examined whether long-term use of melatonin increases the risk of heart failure, is welcome. They use real-life data from the medical records of patients from several countries who were followed up. The method seems appropriate and is typical of drug safety studies.
It was observed that users of melatonin for one year or more had a risk of developing heart failure over five years that was almost double that of those who did not take it. This result could be expressed as follows: of every 100 patients in the study who took melatonin, two who developed heart failure would not have developed it if they had not taken it.
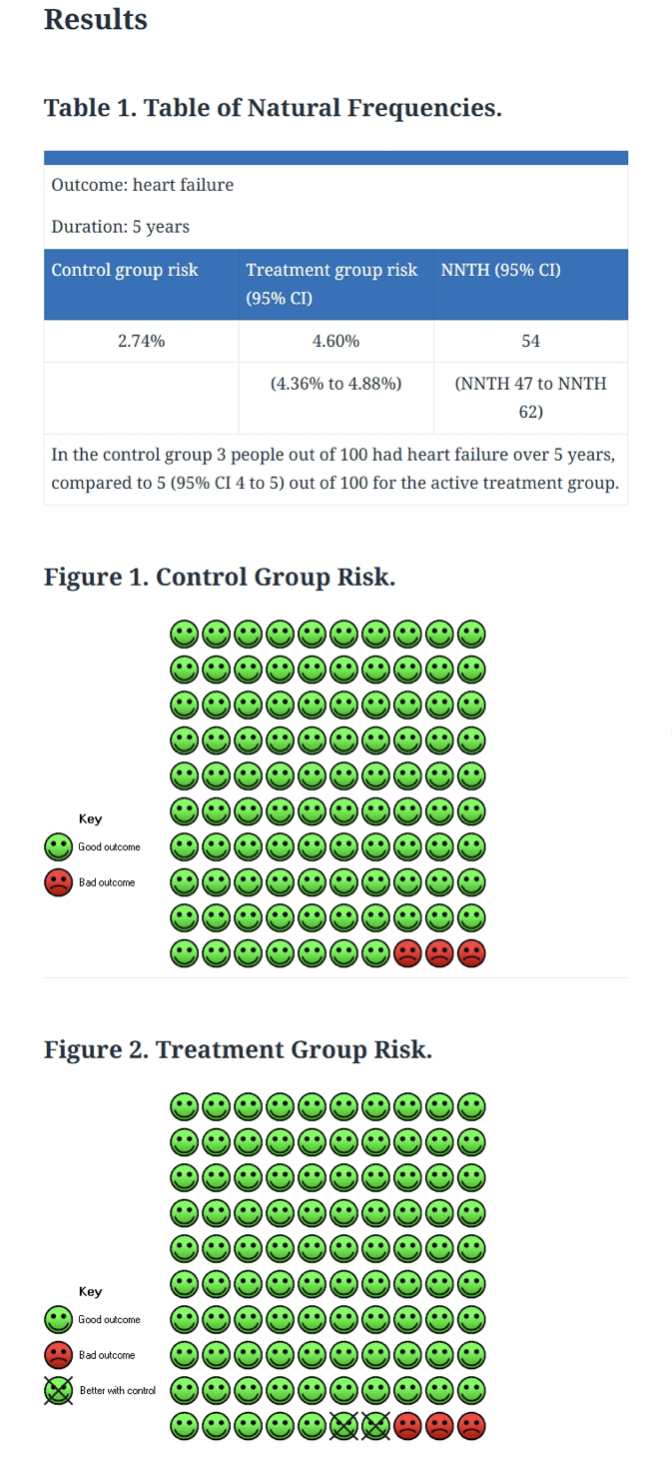
The strength of the study lies in the large number of patients included. It also lies in the fact that each melatonin user was matched with a patient with similar clinical characteristics. However, an inherent limitation of this type of study is that there could be some unknown variable that made patients both more likely to consume melatonin and more susceptible to heart failure.
This study should prompt regulatory bodies to consider whether it would be appropriate to restrict the marketing of melatonin to medicines. The European Medicines Agency should evaluate this study and, if appropriate, include a warning in the information on medicines containing melatonin.
In short, this is an important study on the risks of a product about which there is insufficient information and which should be evaluated by regulatory agencies. It certainly calls into question the claimed cardiovascular safety of melatonin.