Spanish Paediatrics Association
If you are the contact person for this centre and you wish to make any changes, please contact us.
Pediatrician and collaborator of the Advisory Committee on Vaccines, the Spanish Association of Pediatrics and the Spanish Association of Primary Care Pediatrics
Coordinator of the AEP Vaccine Advisory Committee and Primary Care pediatrician
Head of the Pediatrics Unit and coordinator of the Sleep Unit of Hospital Quirónsalud Valencia. Coordinator of the Sleep and Chronobiology Group of the Spanish Association of Pediatrics (AEP).
The Pfizer-BioNTech COVID-19 vaccine has been linked to rare cases of heart inflammation in children and young people. The largest study of these risks in children, published in The Lancet Child & Adolescent Health, concluded that receiving the vaccine is associated with a risk of developing myocarditis or pericarditis within six months of 0.85 additional cases per 100,000 vaccinated children; while after COVID-19 infection, the risk is 2.24 additional cases per 100,000. The study used data from 98% of the British population under the age of 18 (almost 14 million) between January 2020 and December 2022.
Childhood vaccination rates have increased modestly worldwide in 2024, without reaching their pre-COVID-19 pandemic levels, according to data from the WHO and UNICEF. For example, global measles vaccination coverage rose by one percentage point from the previous year, reaching 84% of girls and boys who had received one dose in 2024, compared to 86% in 2019.
An Australian team has conducted a study involving mice and humans that suggests that vaccine boosters would be more effective if administered in the same arm as the previous dose, at least in the short term. However, other recent research has pointed in the opposite direction. The results are published in the journal Cell.
Antimicrobial resistance caused around 5 million deaths worldwide in 2019. The use of vaccines has the potential to reduce these deaths - 515,000 fewer deaths per year - according to a report published by the WHO. The work focused on 24 pathogens and 44 vaccines, licensed by regulatory agencies, in clinical development or in development. By counting existing vaccines alone, antibiotic use could be reduced by 142 million daily doses per year.
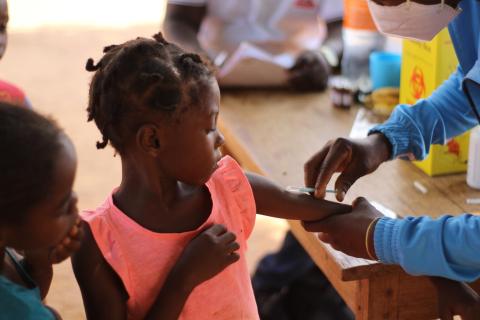
Global childhood immunisation coverage stagnated in 2023, with 2.7 million more children unvaccinated or under-vaccinated than at pre-pandemic levels in 2019. This is one of the data published by the World Health Organization (WHO) and UNICEF in the World Health Organization's Worldwide Estimates of National Immunisation Coverage (WUENIC), which captures global vaccination trends against 14 diseases. More than half of unvaccinated children live in 31 countries with fragile and conflict-affected environments.

Nirsevimab substantially reduced hospitalizations of babies from late September to late December 2023 in Galicia, according to a study published in The Lancet Infectious Diseases. This monoclonal antibody is administered to babies to prevent lower respiratory tract illness caused by respiratory syncytial virus (RSV). In Galicia, over 9 out of 10 babies received nirsevimab, which, according to the research, reduced the risk of hospitalizations for RSV-related respiratory illness by over 80%.
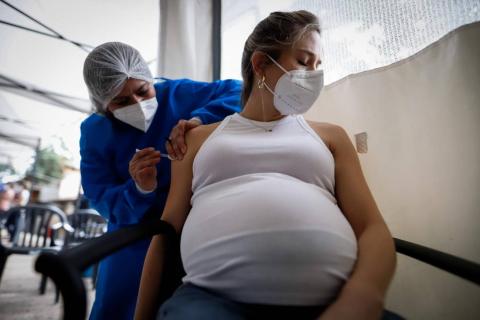
The NEJM publishes the results of a phase III clinical trial conducted by GSK that had to be suspended due to a safety signal. The study shows that newborns of vaccinated mothers had a lower risk of experiencing severe events associated with the respiratory syncytial virus (RSV), but also a higher risk of being born prematurely.

Toledo and Alicante are suffering the first outbreaks of measles recorded in Spain since the pandemic, El País reported today. In total, 15 cases have been confirmed since 1 January, of which seven are imported and eight autochthonous.

The European Medicines Agency (EMA) has recommended marketing authorisation in the European Union for Abrysvo vaccine to protect against respiratory syncytial virus (RSV) disease in infants up to six months of age. It is the first vaccine of its kind indicated for passive immunisation of newborns through administration to the mother during pregnancy. It is also indicated for people over 60 years of age. The European Commission now has to decide on its EU-wide marketing authorisation.
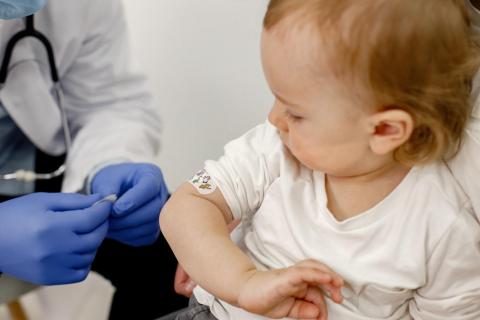
Researchers have analysed the efficacy of the meningococcal serogroup B vaccine (4CMenB, Bexsero) in more than 1,500 children under five years of age in Spain. The research, published in the New England Journal of Medicine, shows that full vaccination was effective in preventing invasive meningococcal disease of both serogroup B and other serogroups in this child population. The vaccine, which had been sold privately in Spain since 2015, had already been included by several autonomous communities in their vaccination schedules. Last December, the Interterritorial Council of the National Health System (CISNS) approved its inclusion in the vaccination schedule for the whole of Spain.